Research of the connective tissue dysplasia effect on the nature and quality of human teething in the late postpartum period of ontogenesis
Downloads
Abstract
It was necessary to study the quality of the hard tissues of teeth 38, 48 and the lower jaw in the projection of these teeth in the late period of postpartum ontogenesis.Material and methods.
The research involved 102 male patients, 76 with connective tissue dysplasia (CTD) and 26 without CTD. They were divided into the groups by age: 31—40, 41—50, 51—60 years old. All of them had one intact tooth 38 and 48 extracted for medical reasons. A fragment of the alveolar part of the lower jaw in the projection of teeth 38 and 48 was taken too at the same time. We analyzed condition of crown and root systems of extracted teeth 38, 48, densitometric density of mineral component of teeth enamel, sizes of enamel prisms, spatial organization of collagen fibers of bone tissue, dimension characteristics of bone plates and mineralization centers of lower jaw.
Results.
High optical density values are observed at the age of 31—40 years (U=2.0602, p=0.0476 relative to the 41—50 years group), at the age of 51—60 years (U=3.6029, p=0.0239 relative to the 41—50 years group), at the age of 41—50 years values are reduced (U=1.0628, p=0.05291) in CTD. Despite the increase in the optical density of the lower jow at the age of 31—40 years with CTD in the points m1vl (rs=0.954, p=0.047) and m2al (rs=0.871, p=0.035) after 40 years there is a decrease in mineral density, where total areas of hypomineralization appear. At the ages of 41—50, 51—60 years, pronounced sclerosis and deformation of demarcation elements are observed at the border of connective tissue structures and periosteum, at 31—40 years these changes are expressed moderately. At the age of 31—40 years the level of lamina delamination is single, after 40 years it is multiple.
Conclusion.
Morphological changes in bone tissue and the slow rate of maturation of teeth 38, 48 are a barrier to their correct and harmonious teething after 30 years.
Key words:
teething, enamel and bone quality, connective tissue dysplasia, ageFor Citation
Introduction
Connective tissue dysplasia (CTD) is a large group of hereditary conditions leading to mutations in different genes, manifesting different clinical phenotypes, ultimately the appearance of humans, has recently attracted special attention of doctors of various medical specialties [1—5]. Systemic connective tissue pathology involves all organs and tissues, including the maxillofacial region (joint hypermobility, changes in the periodontium, salivary glands, dental bite and dentition disorders) [1—3]. In connective tissue dysplasia, the rate of maturation of the mineral component of tooth enamel and the morphostructural characteristics of bone tissue are reduced at a young age, which in turn is reflected in the quality parameters of enamel and bone tissue in later age, determining its rigidity, exposure to pathological conditions of the dentoalveolar apparatus [6—10]. These changes disrupt the normal process of development and eruption of human teeth, preventing the coherence of the genetically determined complex of physical and chemical processes occurring in the enamel and bone tissue [7, 11—17].
The prevalence of connective tissue dysplasia in the population is high and its manifestations in the human body are highly diverse with phenotypic features and genetic patterns both among the whole genus and within a single dentition of an individual [1—3, 7]. Undoubtedly, these problems alter the course, nature of physiological and pathological processes in the maxillofacial region, which is determined by the general laws of nature and evolutionary unity of the organism, eventually in connective tissue dysplasia, characterized by early acceleration changes [2, 3, 18]. This leads to premature or late tooth eruption, which negatively affects the functional and aesthetic capacity of the masticatory apparatus [6—8, 19—22]. In view of the above, the known theories of teething, which reflect the course of these processes under physiological conditions, can be questioned [23]. Summarizing the results of past studies, it can be argued that the mechanisms of tooth eruption have not been sufficiently studied in the scientific world, resulting in the main two theories describing the processes of development and eruption of human teeth, occurring under the influence of physico-chemical factors in the tooth itself or in the surrounding bone tissue, where there is the growth of bone marrow spongy substance of the alveolar ridge, the appearance of specific cellular activity of osteoclasts, osteoblasts [23, 24]. The authors of this model indicate that osteoclastic resorption and simultaneous regeneration occur in bone tissue when functional capacity is not impaired [24]. The search for the mechanisms and nature of tooth eruption in connective tissue dysplasia can be considered a relevant and demanded topic for basic and applied dentistry.
Objective was to study the quality of the hard tissues of teeth 38, 48 and the lower jaw in the projection of these teeth in the late period of postpartum ontogenesis.
Material and methods
The study group consisted of 76 male patients with connective tissue dysplasia, 26 patients without connective tissue dysplasia (comparison group) who applied to the general dentistry department of the City Clinical Dental Polyclinic No.1 of the Omsk Region for the removal of retained tooth 38 or 48 for orthodontic or orthopedic reasons. Comprehensive assessment of the definition of CTD was carried out using diagnostic tables and coefficients, calculated using the Kulbak criterion. Skeletal phenotypic features (asthenic type of constitution, dolychomyelia, arachnodactelia, tower skull, hypertelorism, sandal cleft, vagus or valgus deformity of legs) were determined in all examined patients, great attention was paid to detection of dental stigmas (micro dentition, shovel teeth, trems, diastems, bite disorders, short bridles). All those examined were divided into 3 age groups: 31—40, 41—50, 51—60 years old. To investigate the inorganic component of tooth enamel, a densitometric evaluation of its optical density was performed using computer tomography software from Kodak Dental Systems (Trophy 2000). Regression lines were determined in the program, which are the criteria for identifying patients with low radiological density and low level of maturation and mineralization of dental hard tissues at two points e1b — apex of the cusp of enamel, e2d — cervical area (see CT on Fig. 1; patent RU #2718300 and #2718280, effective from 07.10.2019).
Preparation of tooth samples 38, 48 for atomic force microscopy (AFM) and electron microscopy was carried out by dosed grinding and polishing of enamel to purity class 14 under control of the depth of ground tooth enamel tissues using a dental depth gauge with accurate measurements to 1 µm (patent RU #187021, effective from 02.07.2018). After mechanical processing of the slides, the preparation was cooled with distilled water, dried using a propane burner at 36°C, etched with 37% orthophosphoric acid and finally washed under distilled water (patent RU #2702903, effective from 14.03.2018).
An alveolar fragment of the lower jaw was fixed in 10% formalin and 5% trichloroacetic acid solution for histological examination. Trichloroacetic acid solution was used as a fixative and decalcifying fluid; sharp swelling of collagen structures was taken into account. Decalcification was performed with 0.1 normal hydrochloric acid solution in saline. The readiness test was determined when the lower jaw fragment bent easily under finger pressure. After decalcification, the bone fragment was washed with a final neutralisation of residual acid. Paraffin sections prepared according to the standard technique were stained with hematoxylin-eosin.
The tooth enamel ultrastructure was examined using a Solver Pro scanning probe microscope (NT-MPT, Russia). Computer processing of AFM-image samples was performed using the Image Analysis NT-VDT software. As a result, the shape, surface, packing density, distance between enamel prisms of teeth 38 or 48 were analyzed. The data were processed using variance statistics, using Mann-Whitney (U) and Spearman (rs) tests.
Results and discussion
The age-related dimorphism of human teeth has been proven by now. Objective evaluation of morphometric and morphological parameters of teeth is necessary in a comprehensive study of age-related variability of the maxillary apparatus, as well as reduction processes, which occur differently in age periods.
In our observations, the root system in the groups with and without CTD had low variability at ages 31—40, 41—50, 51—60 (rs=0.281, p=0.083), the number of roots did not exceed two (31—40 years — 70%; 41—50 years — 80%; 51—60 years — 70%), no root fusion was observed at these ages. In the group with CTD at 31—40 years of age there were only isolated observations of 3-rooted (less than 2%) and 4-rooted teeth (less than 2%) with anomalous crooked structure, curvature, but we found no correlation in the studied indicators (rs=0.154, p=1.014). Morphometric parameters of the root system reflect the sufficient level of development and formation of the studied teeth (Table 1).
| Indicator | Age (years) | |||||
| 31—40 | 41—50 | 51—60 | ||||
| without CTD | with CTD | without CTD | with CTD | without CTD | with CTD | |
| Number of roots | 1.7±0.2 | 1.9±0.2 | 1.6±0.3 | 1.5±0.2 | 1.8±0.2 | 1.9±0.3 |
| Root length (mm) | 10.9±1.3 | 10.7±1.1 | 10.8±0.8 | 10.9±1.1 | 10.6±0.5 | 10.7±0.6 |
The optical density of the mineral component of the enamel of the studied teeth as measured by CT densitometry increases with age in the two groups (as measured by e1b: 31—40 years: rs=0.485, p=0.034; 41—50 years: rs=0.497, p=0.032; 51—60 years: rs=0.638, p=0.027; for e2d: 31—40 years: rs=0.689, p=0.024; 41—50 years: rs=0.682, p=0.025; 51—60 years: rs=0.522, p=0.036). In CTD, its values were high at ages 31—40 years (U=2.0602, p=0.0476 relative to 41—50 years group), at ages 51—60 years (U=3.6029, p=0.0239 relative to 41—50 years group), at ages 41—50 years values were reduced (U=1.0628, p=0.05291). In the group without CTD, mineral density shows high values without at 41—50 years of age (U=2.0388; p=0.0315 relative to the 31—40 years group), at 51—60 years of age (U=3.6029, p=0.0239 relative to the 31—40 years group). Age-specific CT-densitometric values indicate a high level of maturity of the studied teeth in the group without CTD, while in CTD the level of mineral density is reduced (p<0.05).
In the analysis of CT densitometry and histogram, a decrease in bone density was observed in 95% of cases with CTD. Despite an increase in optical density at 31—40 years of age with CTD at m1vl (rs=0.954, p=0.047) and m2al (rs=0.871, p=0.035), a decrease in mineral density occurs after 40 years of age, where total areas of hypomineralisation appear.
In both groups, the lower jaw angle in the area of teeth 38, 48 is over 900, however, in the group with CTD it was close to straight (at age 31—40 years U=5.959, p=0.0092 between group with and without CTD; at age 41—50 years U=4.5187, p=0.0168 between group with and without CTD), after 40 years it was blunter (Table 2). There were no significant differences in the 51—60 years group (p>0.05). This anatomical feature of the lower jaw in the group with CTD had no negative impact on the eruption of the studied 38, 48 teeth.
| Points for measuring the optical density of hard tissues | Age (years) | |||||
| 31—40 | 41—50 | 51—60 | ||||
| without CTD | with CTD | without CTD | with CTD | without CTD | with CTD | |
| e1b tooth enamel (unit) | 827.39±20.21* | 655.11±21.38** | 884.37±24.23* | 611.37±18.83** | 908.12±25.11* | 801.48±22.34** |
| e2d tooth enamel (unit) | 879.97±22.14* | 698.52±25.59** | 902.44±17.26* | 632.13±19.25** | 964.57±23.17* | 804.52±23.08** |
| m1vl lower jaw (unit) | 478.32±18.77* | 404.98±24.21** | 445.67±21.51* | 395.33±25.56** | 419.82±23.18* | 370.15±24.89** |
| m2al lower jaw (unit) | 532.65±15.87* | 460.47±25.92** | 498.69±18.11 | 447.6±25.31** | 491.14±19.88 | 424.58±26.72** |
| ams angle (°) | 138.5+3.3 | 99.2+3.8** | 139.9+3.8 | 110.5+5.7** | 143.5+3.7 | 115.7+4.9** |
| amd angle (°) | 137.1+3.1 | 102.3+3.6** | 137.8+3.5 | 127.4+3.7** | 146.0+4.2 | 132.6+4.1** |
| * — statistically significant difference between age groups (p<0.05); ** — statistically significant difference patients with and without CTD (p<0.05). | ||||||
At 31—40, 41—50 years without CTD, the presence of roughness and irregularity on enamel prisms was not observed; at similar ages with CTD, roughness and irregularity was minimal (Fig. 2a and 2b, Fig. 3a and 3b). At 51—60 years of age, the relief of enamel prisms was completely absent in both groups. We detected no branching into multiple prisms or their fusion in both groups studied (Fig. 2c and Fig. 3c).
The mineral component peaks at 31—40 years of age, while the non-CTD group peaks at 51—60 years of age. Human dental enamel in both groups contains large enamel prisms; in the group with CTD, small enamel prisms of ugly shape and configuration are more common; in the group without CTD, small prisms are almost invisible in the field of view of the atomic force microscope. In the group with CTD, there is a large amount of organic matrix and large distances between enamel prisms; in the group without CTD, the prisms are more densely packed. The magnitude of the distance between enamel prisms decreases with age, in the group with CTD up to 40 years, without CTD up to 30 years, and does not change thereafter. At ages 31—40, 41—50, 51—60, we observed the presence of prismatic envelope as a barely visible rim interrupted in some areas of enamel prisms in the group without CTD (Table 3).
| Parameter | Age (years) | |||||
| 31—40 | 41—50 | 51—60 | ||||
| without CTD | with CTD | without CTD | with CTD | without CTD | with CTD | |
| Length of enamel prisms (nm) | 5.58±0.22* | 4.26±0.24** | 6.01±0.26 | 3.82±0.21 | 6.23±0.14 | 3.96±0.27 |
| Width of enamel prisms (nm) | 4.76±0.25* | 4.01±0.21** | 5.12±0.11 | 3.12±0.18** | 5.21±0.27 | 5.28±0.24 |
| Distance between enamel prisms (nm) | 0.53±0.03* | 3.24±0.46 | 0.37±0.04 | 3.33±0.12 | 0.32±0.01 | 3.55±0.22 |
| Prismatic shell size (nm) | less than 1.0 | less than 1.0 | less than 1.0 | less than 1.0 | less than 1.0 | less than 1.0 |
| Interprism gap (nm) | 2.09±0.28 | 3.05±0.52 | 2.06±0.21 | 3.14±0.47 | 1.98±0.17 | 2.74±0.65 |
| * — statistically significant difference between age groups (p<0.05); ** — statistically significant difference patients with and without CTD (p<0.05). | ||||||
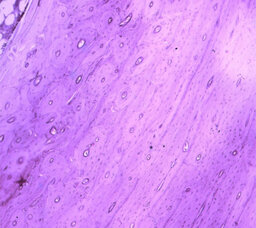
At histological examination of bone tissue at ages 41—50, 51—60 years, at the boundary of connective tissue structures and periosteum, pronounced sclerosis and deformation of delineation elements are observed; at age 31—40 years, the above changes are expressed moderately; in the periosteum proper and compact layer of the lower jaw, changes in bone element structures are observed: at age 31—40 years, the Haversian canals are dilated (Fig. 4, 5) in which pronounced tortuosity and multiplication of vessels are observed, at ages 41—50, 51—60 the Haversian canals are narrowed (Fig. 6), tortuosity and multiplication of vessels are less pronounced (Table 4). In some areas, osteoblasts predominate and osteocyte vacuolisation is evident in the bone tissue proper in all subjects. These changes testify to the balance of destructive and regenerative processes of bone tissue in the projection of undrilled teeth 38, 48. Examination of the compact and cancellous substance of the lower jaw shows gradual changes in the bone structure which is connected with dystrophic transformation of the bone structure of the compact and cancellous substance, with focal thinning at the age of 31—40 years and total thinning at the age of 41—50 and 51—60 years, spongification of the compact plates is observed at all ages (Fig. 7, 8). With the progression of osteoporosis there are numerous large medullary spaces, which are clearly present at age 51—60 years. With increasing age, the bone plates become similar in structure to spongy substance (Table 4).
| Indicator | Age (years) | ||
| 31—40 | 41—50 | 51—60 | |
| Bulk density of the compact substance of the cortical layer (rel. units) | 35.22±3.10* | 28.12±3.90* | 26.53±2.30* |
| Bulk density of cancellous bone (rel. units) | 39.98±6.90* | 34.57±5.50 | 30.91±3.67* |
| Spongy to compact ratio index | 1.13±0.2 | 1.1±0.1 | 1.08±0.2 |
| Average number of Haversian canals per field of view | 4.5±0.2* | 3.8±0.1 | 3.7±0.2 |
| Average number of vessels per field of view | 16.02±2.01* | 14.23±1.57* | 12.57±2.1* |
| Average diameter of Haversian canals | 3.60±0.30 | 3.51±0.2 | 3.44±0.3 |
| * — statistically significant difference between age groups (p<0.05). | |||
At the age of 41—50 years, the spongy substance begins to loosen along with the loosening of the compact bone substance. Bone marrow spaces increase with age, bone beams become thin and deformed, after 40 years of age they become sinuous short due to partial resorption, which leads to the merging of individual bone spaces into large cavities. At the age of 31—40 years, the bone spaces are round and oval in shape; after 40 years, they become polygonal and sinuous.
Atomic force microscopy showed that collagen fibrils form the basis of the bone matrix of the examined subjects. In the lamellar bone tissue in the examination area, the collagen fibres are insufficiently oriented. At all ages, we observe multiple splitting of the laminae, which disturbs the unity and harmony of the fibrous structure of the entire bone tissue. At the age of 31—40 years, lamina delamination is observed at the level of single fibrils, and the level of lamina delamination is insignificant. After the age of 40, the lamina delamination is multiple and also affects the majority of the fibrils. These bone morphological changes in the area of teeth 38, 48 are a barrier to their correct and harmonious eruption.
Straight bone beams predominate in all examined groups. At the age of 31—40 years, beams with irregular shapes and chaotic arrangement of bone plates were found only in the lower zone, after 40 years were found everywhere. The consequence of this is the presence of a large number of cavities, which leads to a change in the bone mineral content and bone plate formation and a reduction in the amount of mineral content per unit bone volume. All this leads to the fact that after 30 years of age, we observe morphological signs of a pronounced erosion of the zoning of the bone structures in the periosteum and compact layer itself, the bone beams become deformed, uneven and thinning.
In all examined groups, there are increased foci of basophilic staining in the thickness of the bone beams due to irregular calcium distribution of chondromatous degeneration; atrophic destructive changes predominate in the bone tissue. The compact bone plates are irregularly calcified, more weakly in the marginal zones. There are girdles with osteoid layering and areas filled with loose fibrous tissue, sometimes with myxomatous foci. The occurrence of myxomatous areas and voids in the form of luminescence foci leads to an abnormal bone mineralisation process with an increase in density only in the apical part against the background of a general decrease in bone mineralisation.
According to the results of the given research, it has been established that in case of connective tissue dysplasia, the eruption process of teeth 38, 48 is suspended in conditions of low hypomineralisation of dental hard tissue and pronounced hypomineralisation of the lower jaw mineral component, despite favourable anatomical conditions in the form of a change in the bone tilt angle towards its bluntness. After 30 years of age, the presence of irregularities and roughnesses on the surface of the enamel prisms is found in minimal numbers, the enamel prisms have a sufficient level of packing, in insignificant numbers ugly and irregularly shaped enamel prisms are traced, indicating the presence of local areas of hypomineralisation. After 40 years of age, in the connective tissue structures and periosteum of the lower jaw, in projection of retained teeth 38, 48, marked sclerosis and deformity of demarcation elements are observed; in age 31—40 these changes have moderate character. Changes in bone tissue testify to the balance of destructive and regenerative processes in the projection of retained teeth 38, 48. With the progression of osteoporosis, numerous large bone laminae appear which are vividly presented at the age of 51—60 years. With increasing age, the bone plates become similar in structure to the spongy substance. After the age of 30 in connective tissue dysplasia, the bone plate's delamination is total, manifested by the delamination of multiple fibrils. These changes have a significant impact on the poor orientation of the collagen fibres and the lack of harmony in the fibrous structure of the lower jaw after 40 years of age.
Conclusions
Morphometric parameters of crown and root systems of teeth 38, 48 indicate the sufficient level of their formation. Research of ultrastructure of a mineral component of teeth enamel and a lower jaw by atomic force microscopy specifies the expressed age dimorphism of their structure, in a lower jaw on progressing sclerotic and degenerative changes with deformation and cleavage of bone plates at a level of the majority of fibrils characteristic for osteoporosis. Investigated changes at dysplasia of connective tissue in teeth 38, 48 and bone tissue are characterised by insufficient rate of maturation of dense tissues of the maxillofacial region by the presence of hypomineralised areas. Unfavourable morphofunctional conditions reduce the probability of eruption of teeth 38, 48 after the age of 30.
References
- Grigorovich E.Sh., Polyakova R.V., Samokhina V.I. Peculiarities of dentistric status of adults and children associated with various somatic diseases on the background of connective tissue dysplasia. Pediatric Dentistry and Profilaxis. 2018; 2 (65): 32—7 (In Russ.). eLIBRARY ID: 35290313.
- Orekhova L.Yu., Churilov L.P., Stroev Yu.I., Alexandrova A.A. Systemic connective tissue dysplasia as a common problem in general medicine and dentistry. Parodontologiya. 2010; 1 (54): 8—14 (In Russ.). eLIBRARY ID: 15244672.
- Sesorova I.S., Shnitkova E.V., Lazorenko T.V., Zdorikova M.A., Podosenkova A.A. Connective tissue dysplasia as a risk factor for the development the dentalveolar of anomalies. Modern problems of science and education. 2016; 6: 182 (In Russ.). eLIBRARY ID: 27694988.
- Statovskaya E.E. The treatment regimen basis of pathological states of masticatory apparatus in patients with dysplasia of connective tissue. The Dental Institute. 2009; 3 (44): 44—5 (In Russ.). eLIBRARY ID: 13058627.
- Kravtsov Y.A., Yavorskaya M.V. Clinical examples of connective tissue dysplasia in patients with different age with surgical pathology. Scientific Review. Medical sciences. 2017; 5: 49—57 (In Russ.). eLIBRARY ID: 29826100.
- Vagner V.D., Konev V.P., Korshunov A.S. Age changes in mineral component and organic matrix of human teeth enamel by electronic and atomic-power microscopy methods. Clinical Dentistry (Russia). 2019; 91 (3): 4—6 (In Russ.). eLIBRARY ID: 41188345.
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Skurikhina A.P., Bondar A.A. Comparative assessment of the rate and quality of the enamel mineral component maturation of human teeth with connective tissue dysplasia in the late postpartum period of ontogenesis. The Dental Institute. 2020; 4 (89): 72—3 (In Russ.). eLIBRARY ID: 44287055.
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Skurikhina A.P., Bondar A.A. Research of the structure of teeth enamel mineral component in connective tissue dysplasia by densitometry and atomic force microscopy in the late postpartum ontogenesis period. Clinical Dentistry (Russia). 2020; 4 (96): 19—24 (In Russ.). eLIBRARY ID: 44476495.
- Konev V.P., Moscovskiy S.N., Shestel I.L., Shishkina Yu.O., Korshunov A.S. Investigation of the mineral component and organic matrix of bone tissue using methods of atomic-power microscopy. Forensic medicine. 2018; 4 (1): 22—5 (In Russ.). eLIBRARY ID: 36907457.
- Shen L., de Sousa F.B., Tay N.B., Lang T.S., Kaixin V.L., Han J., Kilpatrick-Liverman L.T., Wang W., Lavender S., Pilch S., Gan H.Y. Deformation behavior of normal human enamel: A study by nanoindentation. J Mech Behav Biomed Mater. 2020; 108: 103799. PMID: 32469721.
- Leont’ev V.K. Tooth enamel as biocybernetic system. Moscow: GEOTAR-Media, 2016. P. 72 (In Russ.). eLIBRARY ID: 26074164.
- Poggio C., Ceci M., Beltrami R., Lombardini M., Colombo M. Atomic force microscopy study of enamel remineralization. Ann Stomatol (Roma). 2014; 5 (3): 98—102. PMID: 25506414.
- Jheon A.H., Seidel K., Biehs B., Klein O.D. From molecules to mastication: the development and evolution of teeth. Wiley Interdiscip Rev Dev Biol. 2013; 2 (2): 165—82. PMID: 24009032.
- Huang X.-F., Chai Y. Molecular regulatory mechanism of tooth root development. Int J Oral Sci. 2012; 4 (4): 177—81. PMID: 23222990.
- Koldehoff J., Swain M.V., Schneider G.A. The geometrical structure of interfaces in dental enamel: A FIB-STEM investigation. Acta Biomater. 2020; 104: 17—27. PMID: 31917293.
- Beniash E., Stifler C.A., Sun C.-Y., Jung G.S., Qin Z., Buehler M.J., Gilbert P.U.P.A. The hidden structure of human enamel. Nat Commun. 2019; 10 (1): 4383. PMID: 31558712.
- Nurbaeva M.K., Eckstein M., Feske S., Lacruz R.S. Ca2+ transport and signalling in enamel cells. J Physiol. 2017; 595 (10): 3015—3039. PMID: 27510811.
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Skurikhina A.P., Bondar A.A. Comparative assessment of the rate and quality of enamel mineral component maturation of human teeth with connective tissue dysplasia in the early postpartum period. Clinical Dentistry (Russia). 2021; 1 (97): 6—11 (In Russ.). eLIBRARY ID: 44847622.
- Dean M.C., Humphrey L., Groom A., Hassett B. Variation in the timing of enamel formation in modern human deciduous canines. Arch Oral Biol. 2020; 114: 104719. PMID: 32361553.
- Pandya M., Diekwisch T.G.H. Enamel biomimetics-fiction or future of dentistry. Int J Oral Sci. 2019; 11 (1): 8. PMID: 30610185.
- Hogg R.T., Richardson C. Application of image compression ratio analysis as a method for quantifying complexity of dental enamel microstructure. Anat Rec (Hoboken). 2019; 302 (12): 2279—2286. PMID: 31512393.
- Carreon A.H., Funkenbusch P.D. Nanoscale properties and deformation of human enamel and dentin. J Mech Behav Biomed Mater. 2019; 97: 74—84. PMID: 31100488.
- Postolaki A.I. Morphodynamic model of tooth eruption mechanism in humans. National Association of Scientists. 2015; 11-1 (16): 164—8 (In Russ.). eLIBRARY ID: 29243086.
- Vatlin A.G. Development of computer program of determination of biological age on number of cutting constant teethes and integral factor eruption. Morphological statements. 2006; 1-2: 139—42 (In Russ.). eLIBRARY ID: 14808782.