DOI:
10.37988/1811-153X_2021_4_74The influence of zero-gravity on the volume of the augmented in the posterior maxilla bone and the survival of the dental implants placed in this bone
Downloads
Abstract
This article summarizes our experience on survival of dental implants placed simultaneously or delayed with guided bone regeneration and sinus lifting in patients who worked at zero-gravity of orbit.Materials and methods.
We have had three patients with low height and width of the alveolar ridge in the posterior maxilla planned for implant treatment before going to orbit. We augmented the atrophic ridge with guided bone regeneration and sinus lifting. We used “Bio-Oss” (Geistlich Pharma, Switzerland) as a grafting material. The dental implants Xive S plus and AstraTech (Dentsply Sirona, USA) were placed simultaneously or delayed with the procedure. We used CBCT to plan the operations and follow the patients after their return from orbit.
Results.
The patients from our study worked at zero-gravity for 181 days on average. Two of them had a spacewalk of 17 hours 14 minutes on average. CBCT data 6 months after their return from orbit showed a gain of 4.3 to 9.2 mm in height, and 4.0 to 6.5 mm in width. To date, it has been 6,5 years following the loading of the implants: the survival of dental implants is 100%, only one patient had an average 2.5 mm loss of the marginal bone at the implant site. Discussion. Though grafting material for guided bone regeneration and sinus lifting remains under debate, our experience proves the osteoconductive ability of “Bio-Oss” (Geistlich) xenograft as the sole grafting material. It is critical to follow the four principles of guided bone regeneration for a predictable result.
Conclusion.
The volume of the newly created bone remains stable at zero-gravity in a long-term follow-up. Poor quality gingiva around the implant adversely affects the surrounding bone. Sinus lifting can be performed in patients of jobs with the high physical health demands.
Key words:
sinus lifting, guided bone regeneration, dental implant, zero-gravityFor Citation
Introduction
Dental implants are effective medical devices to restore a person's ability to chew or their appearance nowadays. Dental implant survival, functional and esthetic reconstruction following implant treatment are highly dependent on sufficient volume and favorable architecture of the alveolar ridge. Forty percent of the American population loses all teeth in one or both jaws after 60 years old due to complicated decay [1]. It takes 40 days for a dental alveolus to heal [2, 3]. Unloaded alveoli lose bone immediately after heeling. It can be detected 3 to 4 months following tooth loss. Alveolar ridge can lose up to 1.5—2.0 mm high and 40—50% of width 6 to 12 months after tooth extraction [4]. Alveolar ridge deficiency worsens with time without functional load. Usage of removable dentures accelerates and deteriorates this process [5]. The adverse changes of the alveolar ridge bone can make it impossible to place dental implants requiring augmentation procedures to make it higher and broader [6]. Guided bone regeneration (GBR) is one of the procedures to augment the alveolar ridge bone. It employs the concept of promoting the migration of slow-growing osteogenic and angiogenic cells from the adjacent bone marrow into the defect while preventing fast-growing epithelial cells and fibroblasts from occupying that space [7, 8]. A bony defect is filled with bone grafts (autogenous or xenogenic bone or a mixture of both) to serve as an internal framework. The bone graft should be impregnated with blood to form a fibrin clot, which serves as a scaffold for the in-growth of progenitor cells. Platelets of the blood clot collapse, releasing the growth factors that induce osteogenic cells migration and blood vessels ingrowth. Four main principles are needed to be met for the GBR to be efficient: exclusion of epithelium and connective tissue, space maintenance, stability of the blood clot, and primary wound closure [9].
Tatum, Boyde, and James proposed maxillary sinus augmentation to solve vertical bone deficiency in the posterior maxilla [10, 11]. Maxillary sinusitis due to bone graft infection is one of the grave complications of the procedure. It can make a patient unfit for some occupations. Perforation of the maxillary sinus membrane can lead to this severe complication that requires long-lasting treatment. Nevertheless, a lateral window sinus augmentation is a highly effective and predictable procedure in case of adequate diagnostic, patient choice, and good surgical technique [12—14].
When placed into the bone reconstructed with maxillary sinus augmentation or guided bone regeneration, dental implants' success and survival rate can be as high as 97—100% [15]. Surgeons have no unique opinion on which bone graft material has the best effect on bone formation. It is believed that a surgeon's skills and defect characteristics influence the result of the operation to a greater extent than a type of bone graft material [16]. It is also unclear if different bone graft materials affect augmented bone stability.
Available literature has no data representing changes that might occur in the augmented bone under zero gravity conditions and how zero gravity might influence the success and survival rates of dental implants placed into the augmented bone.
This retrospective case series reports clinical and radiographic success and survival rates of dental implants placed after sinus augmentation in patients who had to periodically work in zero gravity conditions.
Materials and methods
Since 2013, unilateral maxillary sinus floor augmentation and guided bone regeneration procedures have been performed in three men patients (48 y.o. mean). All three patients had lost three teeth in the posterior maxilla and required missing teeth with dental implants. Before the treatment, each patient had spent 233 days in near-earth orbit on average. Two of them made spacewalks lasting an averagely of 12 hours and 11 minutes. In all cases, the mean period before the operation after the last space flight was one year and four months. They had deficient high and width of the alveolar ridge not sufficient for dental implants placement. CBCT showed the mean width of the alveolar ridge as follows: patient 1 — 4.7 mm, patient 2 — 3.0 mm, patient 3 — 4.4 mm. The mean high of the alveolar ridge was: patient 1 — 6.4mm, patient 2 — 7.7 mm, patient 3 — 5.0 mm
Maxillary sinus floor augmentation was performed through a lateral window. The incision was made into the gingiva at the top of the alveolar ridge. The incision went into the dentogingival sulcus three teeth medially and onto the maxillary tuberosity distally. In the exposed lateral boney wall was cut a 5x8 mm window to the sinus. The membrane was separated from the sinus floor and lifted 7—9 mm depending on the residual crestal bone height. The created space was filled with 1—2 mm particles of a xenograft Bio-Oss (Geistlich) mixed with auto venous blood only. Autogenous bone was not added to the xenograft. The amount of the blood was just enough to soak and cover the graft. The blood and graft mixture was prepared in advance to let the fibrin clot formation. Moreover, the graft particles glued together in the clot are more easily placed into the sinus than separate ones. We didn't use the collagen membrane to separate the graft from the sinus mucosa or the window from the gingiva.
Horizontal ridge augmentation was made with guided bone regeneration.
For the GBR procedure, we used xenograft prepared in the manner described above. The surface of the alveolar ridge bone was stripped of any residual soft tissue and perforated with a 1.1 mm drill. In two cases, the bone substitute was packed into the bony defect and covered by a Bio-Gide (Geistlich) resorbable membrane; in another one by the non-resorbable membrane (Free-Oss). The latter was fixed to the bone with titanium screws 1.5x4.0 mm. We didn't pin the collagen-derived membranes. In one case, we used a bone block from the oblique line of the ramus of the mandible. We inserted the dental implants Xive S plus (Dentsply) simultaneously with augmentation procedures in one patient. The dental implants were allowed 195 days of integrating time before placing the teeth on them. In two other cases, the dental implants AstraTech (Dentsply) and Xive S plus (Dentsply)) were placed in a two-stage technique 184 days, on average, after the augmentation procedures.
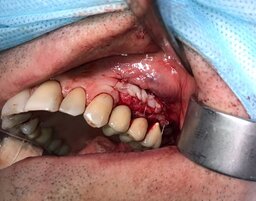
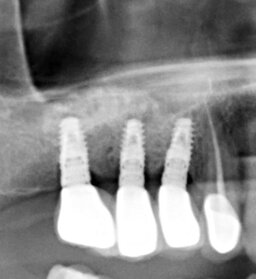
The number of the implants matched the number of the lost teeth. We loaded the implants 152 days after their placement, on average. Two patients required recreation of the attached gingiva around the implants with transplantation of gingiva from the palate (Fig. 1).
Results
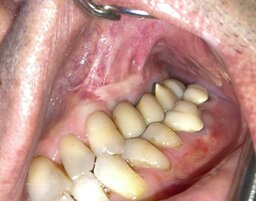
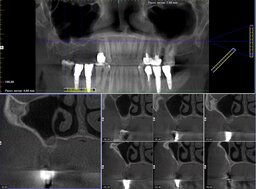
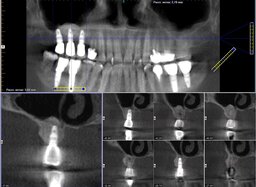
In all cases, the wounds healed primarily. The range of the gained bone volume was 4.3—9.2 mm in height and 4.0—6.5 mm in width (Table). Two patients had metal-ceramic crowns attached to the implants with screws; one patient had his all-zirconia crowns placed on the abutments with dental cement. After loading the implants, all the patients spent an average of 181 days in zero gravity conditions. Two of them had spacewalks lasting 17 hours and 14 minutes on average. Six months after returning from space, the patients were examined by a dentist with a cone-beam computed tomography (CBCT) scanning to check bone resorption around the implants, width, and height of the augmented bone. The implant restorations had no defects. CBCT showed no bone resorption at that time (Fig. 2). Later on, a dentist followed the patients with CBCT once a year. By the time of writing the article, the implants had been loaded for more than six years on average. The implants remained integrated with the bone. Seven years after loading the implants, the patient who didn't have gingivoplasty developed marginal bone resorption measuring 2.3 mm average (Fig. 3). Other patients have no problems. One restoration has a tiny chip that doesn't require replacement.
| Vertical (mm) | Horizontal (mm) | |
| Patient 1 | 9.2 | 6.5 |
| Patient 2 | 6.0 | 4.2 |
| Patient 3 | 4.3 | 4.0 |
Discussion
Guided bone regeneration and maxillary sinus floor lift have been used to augment the maxilla's alveolar ridge for more than 25 years. The procedures are simple and highly predictable in different defects, making them popular among surgeons [17—19]. Having been reports on successful GBR without the membrane [20], it is one of the fundamental components of the procedure [21—23]. Utilizing the bone or bone substitutes without a membrane is associated with unpredictable results and inevitable bone loss in the long term [24, 25].
The xenograft Bio-Oss used in our operations has a remarkable property of becoming part of the ingrowing bone by gradually absorbing with osteoblasts [26—28]. There is no consensus on using xenograft per se. It is believed that its mixture with auto bone has more regenerative potential. The proportion of the xenograft and the auto bone depends on the regenerative property of the bone [19]. In our practice of reconstructing the bone defects of the same characteristics described in the article, we never mix the xenograft with the auto bone. In addition, the platelet-rich plasma did not provide the xenograft additional regenerative potential when compared with the auto venous blood. Strictly following the four main principles of guided bone regeneration guarantees predictable and stable results of the operation. The experience of alveolar ridge augmentation in patients with high professional health requirements described in the article can prove this statement.
Maxillary sinus floor lift is a kind of guided bone regeneration, the difference being the sinus walls serve as a barrier and framework and a source of the osteogenic cells.
The integrity of the maxillary sinus mucosa is a paramount requirement of the operation's success. The inner surface of the mucosa possesses properties compared to those of the periosteum, and its isolation is not consistent with the purposes of the operation. Some authors propose to enhance the mucosa with a collagen membrane before the placement of the bone material. We find this maneuver undesirable and resort to it only to cover minor rips of the mucosa. Meanwhile, there have been reports on the success of the maxillary sinus floor lift without the bone materials, using blood clots solely. These operations were performed with simultaneous placement of the dental implants [29—34]. These data prove the vital role of the intact sinus mucosa, flawless surgical technique, patient's adherence to the restrictions in the post-op period for a successful sinus lift procedure.
Conclusion
The bone created due to the guided bone regeneration and the maxillary sinus floor augmentation has the same properties as the native bone, maintaining volume even in extremal conditions such as zero gravity of Earth orbit. The xenograft Bio-Oss and the collagen membrane Bio-Gide (Geistlich) used in this operation are reliable and ensure predictable results when mixed with auto blood only. Survival of the dental implants placed in this newly created bone is 100% during a 7-year follow-up period despite a long-lasting stay in zero gravity. Our experience proved that maxillary sinus floor augmentation is predictable and safe for occupations with high health requirements, such as astronauts. The patients whose career necessitates infection-free maxillary sinus (pilots, divers, etc.) can be candidates for the procedure.
References
- Marcus S.E., Drury T.F., Brown L.J., Zion G.R. Tooth retention and tooth loss in the permanent dentition of adults: United States, 1988—1991. J Dent Res. 1996; 75 Spec No: 684—95. PMID: 8594092
- Amler M.H., Johnson P.L., Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960; 61: 32—44. PMID: 13793201
- Amler M.H. The time sequence of tissue regeneration in human extraction wounds. Oral Surg Oral Med Oral Pathol. 1969; 27 (3): 309—18. PMID: 5251474
- Schropp L., Wenzel A., Kostopoulos L., Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23 (4): 313—23. PMID: 12956475
- Carlsson G.E., Bergman B., Hedegård B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol Scand. 1967; 25 (1): 45—75. PMID: 5233859
- Lekholm U., Adell R., Lindhe J., Brånemark P.I., Eriksson B., Rockler B., Lindvall A.M., Yoneyama T. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986; 15 (1): 53—61. PMID: 3083006
- Dahlin C., Linde A., Gottlow J., Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81 (5): 672—6. PMID: 3362985
- Becker W., Becker B.E. Guided tissue regeneration for implants placed into extraction sockets and for implant dehiscences: surgical techniques and case report. Int J Periodontics Restorative Dent. 1990; 10 (5): 376—91. PMID: 2098360
- Wang H.L., Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006; 15 (1): 8—17. PMID: 16569956
- Tatum H. Jr Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986; 30 (2): 207—29. PMID: 3516738
- Boyne P.J., James R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38 (8): 613—6. PMID: 6993637
- Misch C.E. The maxillary sinus lift and sinus graft surgery. In: Misch C.E. (ed.) Contemporary Implant Dentistry. Chicago: Mosby, 1999. Pp. 469—495.
- Pikos M.A. Maxillary sinus membrane repair: report of a technique for large perforations. Implant Dent. 1999; 8 (1): 29—34. PMID: 10356454
- Pikos M.A. Complications of maxillary sinus augmentation. In: Jensen O.T. (ed.) The Sinus Bone Graft. Quintessence, 2006. Pp. 103—125.
- Elnayef B., Porta C., Suárez-López Del Amo F., Mordini L., Gargallo-Albiol J., Hernández-Alfaro F. The Fate of Lateral Ridge Augmentation: A Systematic Review and Meta-Analysis. Int J Oral Maxillofac Implants. 2018; 33 (3): 622—635. PMID: 29763500
- Artas G., Gul M., Acikan I., Kirtay M., Bozoglan A., Simsek S., Yaman F., Dundar S. A comparison of different bone graft materials in peri-implant guided bone regeneration. Braz Oral Res. 2018; 32: e59. PMID: 29995064
- Chiapasco M., Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009; 20 Suppl 4: 113—23. PMID: 19663958
- Bhola M., Kinaia B.M., Chahine K. Guided bone regeneration using an allograft material: review and case presentations. Pract Proced Aesthet Dent. 2008; 20 (9): 551—7; quiz 558. PMID: 19113011
- Liu J., Kerns D.G. Mechanisms of guided bone regeneration: a review. Open Dent J. 2014; 8: 56—65. PMID: 24894890
- Clarizio L.F. Successful implant restoration without the use of membrane barriers. J Oral Maxillofac Surg. 1999; 57 (9): 1117—21. PMID: 10484114
- Simion M., Jovanovic S.A., Trisi P., Scarano A., Piattelli A. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int J Periodontics Restorative Dent. 1998; 18 (1): 8—23. PMID: 9558553
- Malmquist J.P. Successful implant restoration with the use of barrier membranes. J Oral Maxillofac Surg. 1999; 57 (9): 1114—6. PMID: 10484113
- Mellonig J.T., Nevins M. Guided bone regeneration of bone defects associated with implants: an evidence-based outcome assessment. Int J Periodontics Restorative Dent. 1995; 15 (2): 168—85. PMID: 8593981
- Urbani G., Lombardo G., Santi E., Tarnow D. Localized ridge augmentation with chin grafts and resorbable pins: case reports. Int J Periodontics Restorative Dent. 1998; 18 (4): 363—75. PMID: 12693423
- ten Bruggenkate C.M., Kraaijenhagen H.A., van der Kwast W.A., Krekeler G., Oosterbeek H.S. Autogenous maxillary bone grafts in conjunction with placement of I.T.I. endosseous implants. A preliminary report. Int J Oral Maxillofac Surg. 1992; 21 (2): 81—4. PMID: 1602165
- Thaller S.R., Hoyt J., Borjeson K., Dart A., Tesluk H. Reconstruction of calvarial defects with anorganic bovine bone mineral (Bio-Oss) in a rabbit model. J Craniofac Surg. 1993; 4 (2): 79—84. PMID: 8324087
- Wallace S.S., Froum S.J., Tarnow D.P. Histologic evaluation of a sinus elevation procedure: a clinical report. Int J Periodontics Restorative Dent. 1996; 16 (1): 46—51. PMID: 8631610
- McAllister B.S., Margolin M.D., Cogan A.G., Buck D., Hollinger J.O., Lynch S.E. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants. 1999; 14 (3): 361—8. PMID: 10379109
- Lundgren S., Andersson S., Gualini F., Sennerby L. Bone reformation with sinus membrane elevation: a new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004; 6 (3): 165—73. PMID: 15726851
- Chen T.W., Chang H.S., Leung K.W., Lai Y.L., Kao S.Y. Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: a 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg. 2007; 65 (11): 2324—8. PMID: 17954333
- Thor A., Sennerby L., Hirsch J.M., Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: an evaluation of 20 patients treated with 44 Astra Tech implants. J Oral Maxillofac Surg. 2007; 65 (7 Suppl 1): 64—72. PMID: 17586351
- Lin I.C., Gonzalez A.M., Chang H.J., Kao S.Y., Chen T.W. A 5-year follow-up of 80 implants in 44 patients placed immediately after the lateral trap-door window procedure to accomplish maxillary sinus elevation without bone grafting. Int J Oral Maxillofac Implants. 2011; 26 (5): 1079—86. PMID: 22010092
- Jensen S.S., Terheyden H. Bone augmentation procedures in localized defects in the alveolar ridge: clinical results with different bone grafts and bone-substitute materials. Int J Oral Maxillofac Implants. 2009; 24 Suppl: 218—36. PMID: 19885447
- Hatano N., Sennerby L., Lundgren S. Maxillary sinus augmentation using sinus membrane elevation and peripheral venous blood for implant-supported rehabilitation of the atrophic posterior maxilla: case series. Clin Implant Dent Relat Res. 2007; 9 (3): 150—5. PMID: 17716259