Optimization of the surface of titanium dental implants of grade 5 alloy by barrier glass ceramic coating
Downloads
Abstract
Pateks — the technology of applying a biocompatible glass-ceramic coating based on silicon, carbon and nitrogen on dental implants by PECVD is developed by research and production company Plasmacentre and Pavlov University (St. Petersburg, Russia). Purpose of the research — to evaluate the success of optimization of the surface of doped titanium endosseous dental implants which may be achieved with our glass-ceramic barrier coating technology using physicochemical, cytological, spectrophotometric and biological researches. The main task is to carry out a comparative assessment of the studies’ results between dental implants with and without Pateks glass-ceramic coating (doped with titanium alloy).Materials and methods.
Titanium implants (grade 5) from BioMed were used in this research. Some of them were coated with Pateks according to our technology. A human lung fibroblast cells culture was used for the cytological study. Spectrophotometric studies were carried out on atomic absorption spectrophotometer and a atomic absorption spectrometer KVANT-Z.ETA (Russia). Biological studies were carried out on 6 Chinchilla rabbits.
Findings.
Pateks-coated implants demonstrate advantage in comparison with non-coated ones in certain physicochemical parameters such as decrease of defects’ area in the initial surface of titanium implants which is formed on the previous stages of surface shaping and processing, providing higher surface hydrophilicity, increasing of dielectric characteristics and higher pH value. Pateks coating is safe for fibroblast monolayers and does not inhibit respiratory processes in them. Applying of Pateks glass-ceramic coating with a thickness of 0.5 μm reduces the content of aluminum and vanadium ions in the model medium by almost 2 times and provides a barrier that helps to reduce the negative biological effect of these ions on peri-implant tissues. There is a reduction in the osseointegration time of experimental coated titanium implants in a study on rabbits.
Key words:
dental implant, bioinertness, coating, surface, siliconFor Citation
Introduction
The dental market offers a variety of metal dental implant systems. All of them differ in the shape of the intraosseous part, manufacturing technology and surface treatment [1—3]. The optimal material for dental implants is titanium [4—6]. Alloying titanium is to a greater extent a necessity for carrying out various methods of modifying the surface of the intraosseous part, as it increases the manufacturability of pure titanium by reducing its plasticity.
The most common dental implants in Russia made of grade 5 titanium alloys (Ti6Al4V, Russian grade — VT6). This alloy (ISO 5832/3: 2016, GOST 19807-91) contains up to 6.8% aluminum and up to 4.5% vanadium. Some manufacturers use grade 4 titanium alloy (American standard ASTM F67: 2013). According to regulatory documents, it is undoped metal without of vanadium and aluminum inclusions, however, in terms of physical and mechanical characteristics, it is inferior to grade 5 alloy.
The study of installed titanium dental implants showed that diffusion processes take place on their oxide surfaces, resulting in detection of metal ions in the peri-implant tissues [7, 8]. Other studies demonstrate that the formed oxide film is very thin, 1—10 nm, so it does not protect the human body from the toxic effects of aluminum and vanadium ions [9, 10].
Vanadium ions realize their cytotoxicity in various ways. It has been proven that they affect the functions of certain enzymes: ATPases, protein kinases, ribonucleases, and phosphatases [11]. It has also been shown that vanadium is capable of changing the activity of DNA and RNA enzymes, which determines its mutagenic and genotoxic effects [12]. There is information about the negative effect of vanadium on lipid metabolism [11].
Aluminum ions negatively affect bone tissue metabolism, reduce the rate of mineralization by inhibiting ATP, and are also capable of inhibiting erythropoiesis. Aluminum ions can accumulate in nerve tissues, which promote the germination of severe disorders of the central nervous system [13, 14]. The ability of aluminum ions to accumulate in the brain tissues, liver, kidneys and bones has shown in recent studies [15]. There is an evidence of the aluminum toxic effect on mineral metabolism when its exposure on the implant surface is more than 0.1% [7, 9]. Aluminum negatively affects cell differentiation by competing with magnesium and calcium ions and damages cell membranes [16].
In the study of the most commonly used dental implant systems, an expert group of scientists revealed that most of them has surface contamination [17].
There are a lot of technologies use in the manufacturing of dental implants from doped titanium: milling, sandblasting, passivation, anodizing, electrochemical etching and plasma coating. Each of them has its own disadvantages, which ultimately affects the quality of the treated alloy surface [6—9, 18]. To improve the surface characteristics of certain materials and plasma sterilization the world scientific community is actively working on the research and implementation of plasma technologies, both in medicine in general [19—22] and in dentistry in particular [23—28] as well as on employment of silicon compounds to improve biocompatibility parameters [29—33]. However, to apply various thin-film coatings to dental implants physical vapor deposition (PVD) processes are generally proposed. To implement these technologies, high-tech, complex and large-scale equipment is used, which requires highly qualified personnel, specially equipped space, and additional processing methods. The method of applying our coating is simple and affordable, since it requires small-sized and low-power equipment.
The aim of this research is to evaluate the success of optimizing the surface of doped titanium intraosseous dental implants with the glass-ceramic barrier coating developed by us using physicochemical, cytological, spectrophotometric and biological studies.
Materials and methods
The study material was the Pateks — biocompatible glass-ceramic coating, of our development, based on silicon, carbon and nitrogen. We also used samples of titanium dental implants BioMed (grade 5). Some of them were covered with Pateks using the technology and equipment of our development (Fig. 1). To apply the coating, we used the method of plasma enhanced CVD (PECVD) of our development.
The physicochemical characteristics of the Pateks coating were evaluated in the previous stages of our research, when a number of the most significant characteristics of the surface of dental implants were evaluated, according to the ISIS recommendations. The chemical composition, topography and specific characteristics (fractality, homogeneity, presence of microcracks and foreign inclusions and particles) of the surface of dental implants were assessed by X-ray photoelectron spectroscopy, scanning probe microscopy, and others. Additionally, we investigated the mechanical properties, adhesion to the substrate, water contact angle and wear resistance under the influence of microabrasive particles.
For the cytological study, 8 samples of experimental implants made of grade 5 titanium alloy were used: 4 samples without coating, 4 samples with a preliminary applied glass-ceramic coating with a thickness of 0.5—0.7 μm (Fig. 2). The manifestation of cytotoxic behavior was determined in vitro in accordance with the requirements of Russian standard GOST R ISO 10993-5. For the study, extracts were prepared under sterile conditions. Serum-free cultural alpha-MEM medium (Biolot, Russia) was chosen as a model medium for extracts, it was also used in the course of determining cytotoxicity as a negative control. The samples were placed in individual sterile tubes, then thermostated at +37°C. To determine cytotoxicity, a daily monolayer of the human embryo lung fibroblasts was used. Three 96-well plates were seeded with fibroblasts at a seeding concentration of 20 thousand/ml. During the day, the cells were incubated in a CO2 incubator at +37°C. The tests were carried out on a daily cell monolayer that reached subconfluence. The exposure time in a CO2 incubator was 72 hours. The state of the monolayer and the morphology of the cells were monitored daily using an inverted microscope Unico (USA). A quantitative test was staining with a tetrazolium dye thiazolyl blue (Sigma, USA), which intensity in cells is proportional to their respiratory activity (MTT assay). The analysis results were recorded with a Varioskan plate analyzer (Thermo Fisher Scientific, USA) at a characteristic wavelength of 550 nm.
Spectrophotometric studies were carried out on an atomic absorption spectrophotometer (Germany) and an atomic absorption spectrometer Kvant-Z.ETA (Cortec, Moscow, Russia). The exposure time of titanium in the model environment was 14 days at +37°C. A 0.9% sodium chloride solution was chosen as the model medium.
After receiving the results of a cytological study on the safety of glass-ceramic coating with the permission of the Ethics Commission experimental implants were installed in area of diaphysis and metaphysis of the femur in six rabbits of the chinchilla breed which were chosen for the biological study. In the course of the experiment, animal rights were observed within the framework of the Helsinki Declaration of 1975 and its revision of 2000. The maintenance and use of rabbits complied with the accepted recommendations, the internal requirements and the laws of the Russian Federation (Order of the Ministry of Health of the Russian Federation “On Approval of the Rules of Good Laboratory Practice” dated 01.04.2016, No. 199n; SP 2.2.1.3218-14 “Sanitary and Epidemiological Requirements for the Device, Equipment and Maintenance of Experimental Biological Clinics (vivariums)”). Experimental samples of grade 5 alloy dental implants were installed in the left hind paw, and similar glass-ceramic coated implants were installed in the right hind paw (Fig. 3). In order to assess both contact and distant osteogenesis, experimental titanium implants with a special macrodesign were used, the shape of which on the transverse section on one side has a small depression, which contributes to an increase in the area of distant osteogenesis. After one month 3 individuals were withdrawn from the experiment, the remaining ones were withdrawn after 3 months. Then, histological specimens were prepared with their subsequent staining with hematoxylin and eosin and additionally with azure-eosin Romanowsky staining.
Outcomes and discussion
Physicochemical study
According to the results of the study carried out, the Pateks coating has the following advantages: a decrease in the area of defects in the initial surface of titanium dental implants formed from the previous stages of shaping and processing (Fig. 4), ensuring greater hydrophilicity of the surface (Fig. 5), an increase in dielectric characteristics, as well as pH value. The most significant characteristics from a clinical point of view are presented in Table 1.
| Characteristics | Grade 5 titanium alloy | Glass-ceramic coating |
| Elastic modulus, GPa | 115 | 127 |
| Electrical resistivity, Ohm·m | 106 | 108 |
| Water contact angle, ° | 58 | 36 |
Cytological study
During the entire time of incubation of fibroblasts with extracts from the samples, no changes were noted either in the morphology of the cells or in the state of the monolayer as a whole, as compared to the cells in the negative control. As a result of the study, it was revealed that both types of samples with Pateks coatings have practically no effect on cells, both by 24-hour and 72-hour extracts. Cells in the presence of 72-hour extracts with grade 5 titanium alloy implants also did not differ from control samples (Table 2). Thus, the glass-ceramic coating is safe for the fibroblast monolayer and does not inhibit the respiratory processes in them.
| Material | 24-hour extraction | 72-hour extraction | ||
| percentage of the control group, % | deviation from the control group | percentage of the control group, % | deviation from the control group | |
| Pateks coating | 102.0±2.8 | 2.0 | 103.6±7.1 | 3.6 |
| VT6 alloy | — | — | 98.0±2.4 | 2.0 |
Spectrophotometric study
The content of aluminum and vanadium ions passing into the model medium from grade 5 titanium alloy was determined by atomic absorption analysis (Table 3).
| Chemical element | Hygienic norm | VT6 sample | VT6 sample with Pateks coating |
| Aluminium | 0.50 | 0.09 | 0.05 |
| Vanadium | 0.10 | 0.02 | <0.01 |
Biological study
Based on the results of studies of the coating of our development, a hypothesis has been put forward that the positive physicochemical characteristics of dental implants after applying Pateks coating on them, the barrier properties of the coating and the absence of cytotoxic properties can improve the bioinertness of titanium dental implants made of VT6 alloy and optimize the processes of osseointegration. To test this hypothesis, a biological study was carried out — an experiment on animals, the purpose of which is a comparative assessment of the results of osteogenesis and osseointegration of experimental titanium implants made of grade 5 alloy with a coating and control samples without it. In the course of evaluating the results of a biological study, a comparative analysis of histological preparations was carried out according to 4 signs: vascularization severity, bone regenerate restructuring degree, the number of formed bone trabeculae, fibrosis severity. When evaluating histological sections of all rabbits, the same type of results was obtained.
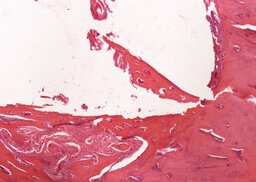
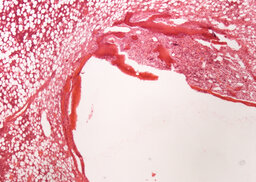
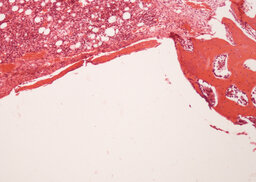
During the analysis of rabbit femurs histological specimens with installed uncoated implants, withdrawn from the experiment a month later, the following was revealed. There are moderate decompression and incomplete reorganization of the bone trabeculae are determined in the zone of tight fit and foci of marginal basophilia along the border between the implant and the bone. In the area of loose fit, a greater amount of fibrous tissue is determined in comparison with the coated implant (Fig. 6, 7).
Fibrous capsule with occasional bone trabeculae is determined in the area of contact with the bone marrow (Fig. 8).
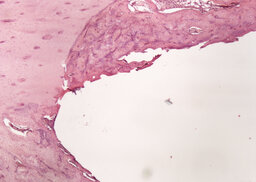
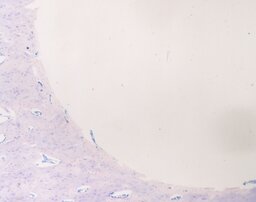
During the analysis of rabbit femurs histological specimens with installed coated implants, withdrawn from the experiment a month later, the following was revealed. In the area of the compact plate, complete compaction and more intense osteogenesis are determined, as evidenced by the formation of a bone with organized beams. In the area of loose fit, there are no foci of basophilia, there is an intense vascularization of the surrounding tissues, the volume of fibrous tissue is a smaller part of the total regenerate compared to an uncoated implant (Fig. 9, 10). In the area of contact with the bone marrow, the fibrous capsule is distinguished by a large number of bone trabeculae (Fig. 11).
According to the data of the histological study of the specimens of rabbits taken out of the experiment after 3 months, there were no significant differences between the results of the experiment with and without coating.
Findings
Reduction of defects on the initial surface of titanium dental implants after coating helps to reduce chemical and bacterial contamination. The creation of a more hydrophilic surface facilitates to decrease water contact angle of the latter, to increase the energy of adhesive interaction and an easier spread of local bone growth factors, as well as protein absorption on the surface of the intraosseous part of dental implants, and increase the rate of blood wettability, the proliferation of fibrin and matrix proteins, what leads to an improvement in bone tissue cell adhesion and contact osteogenesis on the surface of the dental implant. A decrease in electrical conductivity leads to a decrease in the rate of acidification of the tissue environment which is a favorable factor that prevents the emergence of conditions for an increase in aseptic chronic inflammation in the interface area of the dental implant installation, reducing the possible risk of infectious inflammation (mucositis and peri-implantitis).
Application of Pateks glass-ceramic coating with a thickness of 0.5 µm provides a barrier and reduces the content of aluminum and vanadium in the model medium in 2 times. Experimental implants with glass-ceramic coating help to reduce the time of bone tissue compaction in animals compared with uncoated installed implants.
Conclusion
The results of experimental studies of various parameters of the Pateks glass-ceramic barrier coating indicate the successful optimization of the surface of alloyed titanium implants with this coating and its safety, and also substantiate the possibility of applying these coatings in a clinical setting. One of the advantages of applying Pateks coating (SiOCN system) on dental implants made of doped titanium alloy is an increase in bioinertness, a high level of which is typical for grade 4 titanium dental implants, while maintaining those positive physical and mechanical characteristics typical for grade 5 alloy. In the course of research, the choice was justified and the process of applying Pateks biocompatible glass-ceramic coatings was developed. The equipment used for these tasks is small-sized and low-power-consuming, which makes it possible to use in an outpatient clinical setting.
References
- Egorov A.A., Drovosekov M.N., Aronov A.M., Rozhnova O.M., Egorova O.P. Comparative characteristics of materials used in dental implantation. Bulletin of Siberian medicine. 2014; 13 (6): 41—7 (In Russ.). eLIBRARY ID: 22931157.
- Saurabh G. Titanium to ceramic dental implants: A short communication. Journal of Dental Science and Medicine. 2017; 2 (1): 1. DOI: 10.4172/2572-4835.1000116.
- Sultanov A.A., Pervov Yu.Yu., Yatsenko A.K. Physical and chemical properties of implants, their interaction with surrounding tissues and environments of the oral cavity (literature review). Medical newsletter of Vyatka. 2019; 2 (62): 80—6 (In Russ.). eLIBRARY ID: 38213975.
- Trofimov V.V., Fedchishin O.V., Klimenov V.A. The titan, alloys of the titan and their application in stomatology. Siberian Medical Journal (Irkutsk). 2009; 90 (7): 10—2 (In Russ.). eLIBRARY ID: 12966356.
- Rupp F., Liang L., Geis-Gerstorfer J., Scheideler L., Hüttig F. Surface characteristics of dental implants: A review. Dent Mater. 2018; 34 (1): 40—57. PMID: 29029850.
- Hatamleh M.M., Wu X., Alnazzawi A., Watson J., Watts D. Surface characteristics and biocompatibility of cranioplasty titanium implants following different surface treatments. Dent Mater. 2018; 34 (4): 676—83. PMID: 29398110.
- Al Jabbari Y.S., Fehrman J., Barnes A.C., Zapf A.M., Zinelis S., Berzins D.W. Titanium nitride and nitrogen ion implanted coated dental materials. Coatings. 2012; 2: 160—78. DOI: 10.3390/coatings2030160.
- Ehrenfest D.M.D., Coelho P.G., Kang B.-S., Sul Y.-T., Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010; 28 (4): 198—206. PMID: 20116873.
- Novikov S.V., Tamazov I.D., Topolyanskii P.A., Topolyanskii A.P. Use of cold atmospheric plasma in dentistry. Health and Education Millennium. 2018; 20 (1): 124—7 (In Russ.). eLIBRARY ID: 32284233.
- Kamalov R.Ch., Lichota A.N., Kovalenko V.V., Tinkov V.A., Gorobets E.V., Kinchur N.I., Rozova E.V. Comparative analysis of surface structure and her chemical composition at the different systems of dental implants and their influence on the level of sensibilization of organism. Clinical Dentistry (Russia). 2011; 2 (58): 44—48 (In Russ.). eLIBRARY ID: 22653169.
- Tarantin A.V., Zemlyanova M.A. Vanadium essential role and toxic effects. Human ecology. 2015; 12: 59—64 (In Russ.). eLIBRARY ID: 25063260.
- Choi Y., Park K., Kim I., Kim S.D. Combined toxic effect of airborne heavy metals on human lung cell line A549. Environ Geochem Health. 2018; 40 (1): 271—282. PMID: 27888373.
- Shugalej I.V., Garabadzhiu A.V., Ilyushin M.A., Sudarikov A.M. Some aspects of effect of aluminium and its compounds on living organisms. Russian Journal of General Chemistry. 2013; 83: 2633—46. DOI: 10.1134/S1070363213130082.
- Dolara P. Occurrence, exposure, effects, recommended intake and possible dietary use of selected trace compounds (aluminium, bismuth, cobalt, gold, lithium, nickel, silver). Int J Food Sci Nutr. 2014; 65 (8): 911—24. PMID: 25045935.
- Igbokwe I.O., Igwenagu E., Igbokwe N.A. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol. 2019; 12 (2): 45—70. PMID: 32206026.
- Zaitseva N.V., Zemlyanova M.A., Stepankov M.S., Ignatova A.M. Scientific forecasting of toxicity and evaluation of hazard potential of aluminum oxide nanoparticles for human health. Human Ecology. 2018; 5: 9—15 (In Russ.). eLIBRARY ID: 34957396.
- Ushakova A.I. The synopsis of the report on the study of the surfaces of 62 implant models from different manufacturers. Russian Stomatology. 2014; 7 (3): 57—68 (In Russ.). eLIBRARY ID: 22598402.
- Shemtov-Yona K., Rittel D., Dorogoy A. Mechanical assessment of grit blasting surface treatments of dental implants. J Mech Behav Biomed Mater. 2014; 39: 375—90. PMID: 25173238.
- Demnati I., Grossin D., Combes C., Rey C. Plasma-sprayed apatite coatings: Review of physical-chemical characteristics and their biological consequences. Journal of Medical and Biological Engineering. 2014; 34 (1): 1—7. DOI: 10.5405/jmbe.1459.
- Al-Okla S.M., Al Nazwani N.S., Al-Mudarris F.A. Overview of cold atmospheric plasma in wounds treatment. Medical & Clinical Research. 2020; 5 (10): 280—9. DOI: 10.33140/mcr.05.10.04.
- von Woedtke T., Emmert S., Metelmann H.-R., Rupf S., Weltmann K.-D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Physics of Plasmas. 2020; 27 (7): 070601. DOI: 10.1063/5.0008093.
- Shohet J.L. (ed.). Encyclopedia of plasma technology. Boca Raton (FL): CRC Press, 2016. Pp. 328—338. DOI: 10.1081/E-EPLT.
- Arora V., Nikhil V., Suri N.K., Arora P. Cold atmospheric plasma (CAP) in dentistry. Dentistry (Sunnyvale). 2014; 1: 189—93. DOI: 10.4172/2161-1122.1000189.
- Duske K., Koban I., Kindel E., Schröder K., Nebe B., Holtfreter B., Jablonowski L., Weltmann K.D., Kocher T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol. 2012; 39 (4): 400—7. PMID: 22324415.
- Giro G., Tovar N., Witek L., Marin C., Silva N.R.F., Bonfante E.A., Coelho P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J Biomed Mater Res A. 2013; 101 (1): 98—103. PMID: 22826209.
- Cha S., Park Y.-S. Plasma in dentistry. Clin Plasma Med. 2014; 2 (1): 4—10. PMID: 27030818.
- Küçük D., Ercan U.K., Köseoğlu S. The fourth state of matter: Plasma and applications of atmospheric pressure cold plasmas ın dentistry. Yeditepe Dental Journal. 2018; 14(3): 125—36 (In Turkish). DOI: 10.5505/yeditepe.2018.09609.
- Mahrous A., Mohamed S., Ahmed A. The effect of atmospheric plasma-sprayed peek implants on osseointegration. Egyptian Dental Journal. 2018; 64 (1): 733—44. DOI: 10.21608/edj.2018.78085.
- Voronov I.A., Ippolitov E.V., Tsarev V.N. Confirmation of protective characteristics of new coating made of silicon carbide “Shell” in terms of modeling microbial adhesion, colonization and biodestruction based on basic orthopaedic polymers. Clinical Dentistry (Russia). 2016; 1 (77): 60—5 (In Russ.). eLIBRARY ID: 25718070.
- Stephen E. Silicon carbide biotechnology. A biocompatible semiconductor for advanced biomedical devices and applications. Amsterdam: Elsevier Science, 2016. Pp. 251—268.
- Mansurova L.A. Physiological role of silicon. Siberian medical journal (Irkutsk). 2009; 90 (7): 16—8 (In Russ.). eLIBRARY ID: 12966358.
- Fares C., Elhassani R., Ren F., Cabrera A.R., Chai I., Neal D., Hsu S.-M., Esquivel-Upshaw J.F. Color perceptibility and validity of silicon carbide-based protective coatings for dental ceramics. J Prosthet Dent. 2021; S0022-3913(20)30729-0. PMID: 33483139.
- Esquivel-Upshaw J.F., Ren F., Hsu S.M., Dieng F.Y., Neal D., Clark A.E. Novel Testing for Corrosion of Glass-Ceramics for Dental Applications. J Dent Res. 2018; 97 (3): 296—302. PMID: 28922616.