DOI:
10.37988/1811-153X_2021_1_6Comparative assessment of the rate and quality of enamel mineral component maturation of human teeth with connective tissue dysplasia in the early postpartum period
Downloads
Abstract
Objective. It is necessary to study the human enamel prisms size at different periods of postpartum ontogenesis in connective tissue dysplasia by atomic force microscopy.Materials and methods.
The study involved 150 males divided into a group with and without connective tissue dysplasia (each additionally by age: 15—20, 21—30, 31—40 years). Each examined was removed one of the low wisdom teeth (3.8 or 4.8 by ISO). Prepared thin sections of human tooth enamel samples up to grade 14, with maximum preservation of the edges of the enamel prisms within the surface layer. The Image Analysis NT-VDT software was used to analyze the number of faces, shape, length, and width of enamel prisms.
Results.
It was found that at 15—30 years old there is an ordered structure of enamel prisms, a large number of 5-sided forms (50—60%). A permanent form of enamel prisms at 31—40 years old, where the share of 6-sided figures is 30%. With CTD, the growth rate on the plane, which increases the width of the prism, is very similar to the growth rate of length up to 30 years, which leads to a violation of their spatial configuration relative to the organic matrix.
Conclusions.
The maturation of human enamel is strictly individual, depending on the connective tissue dysplasia, which has a negative effect on the rate of maturation, and after eruption can lead to pathological conditions of hard dental tissues.
Key words:
maturation, polymorphism, enamel prisms, connective tissue dysplasiaFor Citation
Introduction
Enamel prisms have a complex multilevel hierarchical system, they change throughout a person's life [1—11]. Before teething, the variability of the mineral component is influenced not only by amelogenins and enamelins, but also by the organic matrix. Its individual structural elements determine the length, width, and thickness of enamel in isolation or in aggregate, forming its three-dimensional configuration [1—5, 9, 12—17]. After the eruption of teeth the mineral component is modified due to the interaction with the oral fluid, represented in different people by an individual quantitative and qualitative composition that determines the development degree, maturation of human tooth enamel, maintaining the longevity of individual groups of teeth and dental row [6, 7, 18, 19]. Thus, the modern dental literature has accumulated enough information about the factors that influence the process of maturation and mineralization of tooth enamel [1—5, 16, 17, 20, 21]. Many authors argue that the full maturation of tooth enamel occurs before eruption, while others point to the maturation of enamel after its eruption [6, 16, 17, 22]. The modern hypothesis testifies to the individual terms of maturation determined in the aggregate by the factors of the external and internal environment of the organism, influencing completeness and formation of the framework and abrasion of the crown part of the tooth [6, 16, 17, 23—25].
Gene mutations cause amelogenesis disruption process with the formation of immature, poor-quality human tooth enamel phenotypes with severe hypoplastic phenomenon in the early stages of tooth maturation [3, 5, 26]. The importance of signaling to the early developmental process is due to the types of genetic defects disrupt it. Genetic alterations that affect early development and maturation processes include transcription factors and signaling molecules. Their receptors lead to hereditary dental agenesis (MSX1, PAX9, AXIN2, EDA genes) or formation of supercomplex teeth (RUNX2, APC) [26].
The active exchange of mineral ions between the enamel and the oral fluid leads to minimal additional calcification of only the surface enamel layer, while in deeper layers after eruption this effect is insignificant — the main frame of the tooth enamel is formed before eruption [1, 2, 4, 6, 11, 19 ]. However, with various forms of amelogenesis impairment, arising at certain development stages and teeth maturation, calcification may not occur, which leads to some clinical phenotypes and the appearance of enamel [3, 5, 26].
There is not enough information in the literature about the nature and rate of early development and maturation of the human teeth enamel mineral component in hereditary conditions that affect the physical and mechanical parameters of the dental enamel. Consequently, the research can be considered relevant.
The study aim was to compare the rate and quality of human dental enamel prisms maturation in the early postpartum period in connective tissue dysplasia (CTD) using atomic force microscopy.
Materials and methods
The study involved 150 men divided into group I with CTD and group II without CTD (each group was additionally ranked by age: 15—20, 21—30, 31—40 years). The CTD presence was determined by the total coefficient of phenotypic manifestations and dental signs using the method of Omsk State Medical University (Omsk, Russia) [27].
One tooth 3.8 or 4.8 was removed for medical indications (from the fracture line in the mandibular angle area for orthodontic indications). The extracted teeth had no contact with oral fluid to exclude its possible influence on the variability of the shape and mineral component structure of the studied teeth. Consecutive and step-by-step preparation of teeth enamel samples was carried out by means of grinding and polishing wheels with bringing of a surface to 14th cleanliness class under the depth control of the grinded teeth enamel tissues by means of a depth gauge stomatological, developed in Omsk State Medical University [28—30].
After mechanical treatment, the thin sections were cooled using distilled water, the preparations were dried using a propane burner at +36°C, and the surface under study was etched with 37% orthophosphoric acid with final rinsing under a stream of distilled water [29, 30]. In this way, it was possible to preserve the facets of the enamel prisms within the enamel surface layer as much as possible.
The obtained thin sections were placed in the field of view of a Solver Pro scanning probe microscope (NT-MPI, Russia); the obtained AFM images were subjected to computer processing using the Image Analysis NT-VDT program. The shape, roughness, length and width of the enamel prisms were analyzed.
Data processing was carried out by methods of variation statistics.
Results and discussion
Enamel prisms in CTD have an irregular, disordered shape with 5-, 6-, and 7-sided figures. The most regular shape of enamel prisms occurs at the age of 31—40 years, at this age the proportion of 6-sided figures is 30% (without CTD, 35%), but there are multiple 7-sided figures (with CTD — 50%; without CTD — 60%). At ages 15—20, 21—30 the enamel prisms are less orderly in structure, 5-sided (15—20 years — 60%, 21—30 years — 50%) and 6-sided figures (15—20 years — 50%, 21—30 years — 40%) are found in great number, 7-sided enamel prism figures are practically not visualized (15—20, 21—30 years — 10%; Table 1).
| Number of faces | Age and group | |||||
| 15—20 years old | 21—30 years old | 31—40 years old | ||||
| I | II | I | II | I | II | |
| Five | 60 | 30 | 50 | 10 | 20 | 5 |
| Six | 30 | 40 | 40 | 50 | 30 | 35 |
| Seven | 10 | 30 | 10 | 40 | 50 | 60 |
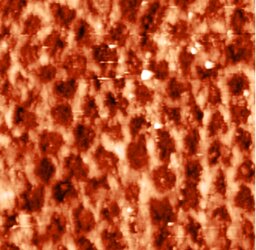
The study of the enamel ultrastructure in patients with CTD by atomic force microscopy showed that the enamel prisms have a rectangular, pointed or square shape with the same shapes at the base. The opposite end of the prisms is pointed, elongated, with which it wedges into the underlying prisms (Fig. 1).
A detailed analysis of the computer images shows that the enamel prisms are wave-shaped in both groups; they are characterized by areas of narrowing and varicosity.
The enamel of a human tooth with CTD at all ages is represented by small prisms, the value of which significantly increases with age and is maximal at age of 31—40 years (χ2=8.24; p=0.0177 relative to the 15—20 years old group; Table 2).
| Age and group | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15—20 years old | 21—30 years old | 31—40 years old | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| I | II | I | II | I | II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Length | 3.46±0.13 | 5.14±0.21 | 3.51±0.12 | 5.23±0.16 | 4.26±0.24* | 5.58±0.22 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Width | 3.25±0.46 | 4.32±0.12 | 3.46±0.33 | 4.45±0.31 | 4.01±0.21* | 4.76±0.25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
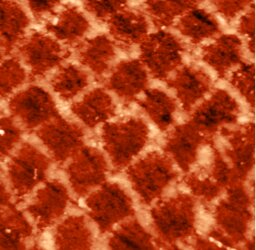
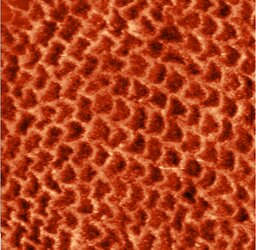
Enamel prisms size polymorphism can be found at all ages with CTD, more often it is expressed at age of 15—20 years (χ2=8.24; p=0.0177 relative to the group of 31—40 years) and at age of 21—30 years (χ2=6,92; p=0.0402 relative to the 31—40 age group), where small-sized enamel prisms have multiple character (see Fig. 1a and b). The main mass at the age of 31—40 years is made up of large prisms, however, small prisms are found in single quantities (Fig. 1c).
It should be noted that enamel prisms are located less densely to each other compared to similar age groups without CTD (Fig. 2).
In both groups, the fastest growth rate of enamel prisms is in the direction increasing prism length at age of 15—20 years (χ2=11.99; p=0.0019 relative to the 31—40 years group) and 21—30 years (χ2=9.09; p=0.0112 relative to the 31—40 years group), but the growth rate in the plane increasing prism width is very similar to the growth rate of length: at age of 15—20 years, the rate of length relative to width in the group rs=0.49 (p=0.721); at 21—30 years, rs=0.21 (p=0.526); at age of 31—40 years, rs=0.692 (p=0.042), which leads to irregular proportions, changes in the shape of enamel prisms resembling different geometric shapes, especially in the 15—20 and 21—30 years old groups. At age of 31—40 years, enamel prisms increase their growth rate in length (χ2=8.21; p=0.0293 relative to the 21—30 age group; U=10.7719; p=0.0049 between group I and II at 31—40 years), they begin to change shape, taking a pyramidal shape, with similar cross-section area at the base and at the apex (Table 2).
A similar structure, the nature of development and maturation during CTD provide a lower enamel hardness, a greater susceptibility to solubility with age, already after the tooth eruption.
As a result of the obtained data analysis on the morphofunctional organization of tooth enamel, we can state the influence of CTD on its maturation to tooth eruption in the deterioration direction of quality and reduction of the rate in the early postpartum period. The method of atomic force microscopy established that in CTD the rate of resorption of the organic matrix is reduced. The characteristic changes lead to slow growth of enamel prisms, with time the growth accelerates, but the prisms take the form of different geometric shapes. Consequently, the conducted research demonstrates the importance of background genetically determined conditions on the formation of the qualitative and mature framework of human teeth enamel, determining the further tooth fate after eruption, when the external environmental factors of the body actively interfere with the metabolic processes.
Conclusions
Summarizing the research of the dental enamel mineral component with CTD in comparison with persons without it, we can state that the structure of the teeth enamel mineral component with CTD is characterized by pronounced polymorphism. Enamel prisms have irregular, disordered shape; it is most expressed in 15—20, 21—30 years with CTD. In CTD, the process of enamel prisms maturation proceeds at a slower pace until the age of 40. This can be judged from the change of their shape, which variability is most pronounced in CTD and characterized by peculiar “deformities” of enamel prisms at the age of 15—20, 21—30 years. Probably, such formations are connected with incomplete process of maturation or with the change of the spatial configuration of the prisms themselves in relation to the organic matrix.
Researches using atomic force microscopy allow the possibility to state the complexity of the enamel maturation mechanisms, which actively occur over a long period of a person's life, end at different age periods and depend on the relationship between the mineral component and the organic matrix. At the same time, the process of enamel maturation in humans is strictly individual, depending on background conditions, such as CTD, which, if adversely affected, influence the degree and rate of enamel maturation, and after eruption can lead to pathological conditions of hard dental tissues.
References
- Vagner V.D., Konev V.P., Korshunov A.S. Age changes in mineral component and organic matrix of human teeth enamel by electronic and atomic-power microscopy methods. — Clinical Dentistry (Russia). — 2019; 91 (3): 4—6 (In Russ.).
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Surkova V.O., Skurikhina A.P., Bondar A.A. Research of the structure of the mineral component of tooth enamel in connective tissue dysplasia by densitometry and atomic force microscopy in the early postpartum ontogenesis period. — Stomatology. — 2020; 99 (6): 7—12 (In Russ.).
- Vagner V.D., Konev V.P., Korshunov A.S. Change of the mineral componente of the teeth enamy during connective tissue dysplasia in the age aspect. — The Dental Institute. — 2019; 83 (2): 20—1 (In Russ.).
- Vagner V.D., Konev V.P., Korshunov A.S., Serov D.O. The research of prismatic shells of human teeth enamel's organic matrix by the atomic-force microscopy method in the postnatal period of ontogenesis. — The Dental Institute. — 2019; 84 (3): 94—5 (In Russ.).
- Konev V.P., Vagner V.D., Korshunov A.S., Serov D.O. The specifics of maturation of impacted teeth enamel's mineral component with connective tissue dysplasia. — The Dental Institute. — 2019; 84 (3): 102—3 (In Russ.).
- Leont‘ev V.K. Tooth enamel as biocybernetic system. — Moscow: GEOTAR-Media, 2016. — P. 72 (In Russ.).
- Shumilovich B.R., Vorob'yeva Yu.B., Malykhina I.E., Chertovskikh A.V. Modern views on the crystal structure of hydroxyapatite and processes age-related changes of tooth enamel (in vitro study). — Journal of Anatomy and Histopatology. — 2015; 4 (1): 77—86 (In Russ.).
- Poggio C., Ceci M., Beltrami R., Lombardini M., Colombo M. Atomic force microscopy study of enamel remineralization. — Ann Stomatol (Roma). — 2014; 5 (3): 98—102. PMID: 25506414
- Cerci B.B., Roman L.S., Guariza-Filho O., Camargo E.S., Tanaka O.M. Dental enamel roughness with different acid etching times: Atomic force microscopy study. — Eur J Gen Dent. — 2012; 1: 187—91. DOI: 10.4103/2278—9626.105385
- Risnes S., Li C. Aspects of the final phase of enamel formation as evidenced by observations of superficial enamel of human third molars using scanning electron microscopy. — Arch Oral Biol. — 2018; 86: 72—9. PMID: 29190456
- Warshawsky H. Organization of crystals in enamel. — Anat Rec. — 1989; 224 (2): 242—62. PMID: 2672889
- Koldehoff J., Swain M.V., Schneider G.A. The geometrical structure of interfaces in dental enamel: A FIB-STEM investigation. — Acta Biomater. — 2020; 104: 17—27. PMID: 31917293
- Pandya M., Diekwisch T.G.H. Enamel biomimetics-fiction or future of dentistry. — Int J Oral Sci. — 2019; 11 (1): 8. PMID: 30610185
- Beniash E., Stifler C.A., Sun C.-Y., Jung G.S., Qin Z., Buehler M.J., Gilbert P.U.P.A. The hidden structure of human enamel. — Nat Commun. — 2019; 10 (1): 4383. PMID: 31558712
- Hogg R.T., Richardson C. Application of image compression ratio analysis as a method for quantifying complexity of dental enamel microstructure. — Anat Rec (Hoboken). — 2019; 302 (12): 2279—86. PMID: 31512393
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Skurikhina A.P., Bondar A.A. Comparative assessment of the rate and quality of the enamel mineral component maturation of human teeth with connective tissue dysplasia in the late postpartum period of ontogenesis. — The Dental Institute. — 2020; 89 (4): 72—3 (In Russ.).
- Vagner V.D., Konev V.P., Korshunov A.S., Kuryatnikov K.N., Skurikhina A.P., Bondar A.A. Research of the structure of teeth enamel mineral component in connective tissue dysplasia by densitometry and atomic force microscopy in the late postpartum ontogenesis period. — Clinical Dentistry (Russia). — 2020; 96 (4): 19—24 (In Russ.).
- Erofeeva E.S., Gileva O.S., Plenkina U.A., Morozov I.A., Svistkov A.L. Experimental investigation of the enamel microstructure on stages of professional bleaching. — Material Science. — 2012; 7 (184): 50—5 (In Russ.).
- Lechner B.-D., Röper S., Messerschmidt J., Blume A., Magerle R. Monitoring demineralization and subsequent remineralization of human teeth at the dentin-enamel junction with atomic force microscopy. — ACS Appl Mater Interfaces. — 2015; 7 (34): 18937—43. PMID: 26266571
- Dean M.C., Humphrey L., Groom A., Hassett B. Variation in the timing of enamel formation in modern human deciduous canines. — Arch Oral Biol. — 2020; 114: 104719. PMID: 32361553
- Nurbaeva M.K., Eckstein M., Feske S., Lacruz R.S. Ca2+ transport and signalling in enamel cells. — J Physiol. — 2017; 595 (10): 3015—39. PMID: 27510811
- Lacruz R.S. Enamel: Molecular identity of its transepithelial ion transport system. — Cell Calcium. — 2017; 65: 1—7. PMID: 28389033
- Eckstein M., Lacruz R.S. CRAC channels in dental enamel cells. — Cell Calcium. — 2018; 75: 14—20. PMID: 30114531
- Carreon A.H., Funkenbusch P.D. Nanoscale properties and deformation of human enamel and dentin. — J Mech Behav Biomed Mater. — 2019; 97: 74—84. PMID: 31100488
- Ortiz-Ruiz A.J., de Dios Teruel-Fernández J., Alcolea-Rubio L.A., Hernández-Fernández A., Martínez-Beneyto Y., Gispert-Guirado F. Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. — Ann Anat. — 2018; 218: 7—17. PMID: 29604387
- Shen L., de Sousa F.B., Tay N.B., Lang T.S., Kaixin V.L., Han J., Kilpatrick-Liverman L.T., Wang W., Lavender S., Pilch S., Gan H.Y. Deformation behavior of normal human enamel: A study by nanoindentation. — J Mech Behav Biomed Mater. — 2020; 108: 103799. PMID: 32469721
- Konev V.P., Moskovskij S.N., Shestel‘ I.L., Korshunov A.S., Abubakirova D.E., Shishkina Yu.O., Smirnov M.V. Screening test of connective tissue displasia by atomic force microscopy. — Certificate of state registration of a computer program RU № 2018617014, effective from 28.04.2018 (In Russ.).
- Korshunov A.S., Muhin A.N., Serov D.O., Konev V.P., Moskovskij S.N., Al'zhanov A.M., Firsova V.O., Kuryatnikov K.N. Dental depthometer. — Patent RU № 187021, effective from 02.07.2018 (In Russ.).
- Shestel‘ I.L., Korshunov A.S., Losev A.S., Shestel‘ L.A., Davletkil‘deev N.A., Konev V.P. The method of making dental preparations for morphological studies of enamel prisms in atomic force (AFM) and inverted microscopes. — Patent RU № 2458675, effective from 04.05.2011 (In Russ.).
- Korshunov A.S., Konev V.P., Serov D.O., Moskovskij S.N. The method of making dental preparations for morphological studies of enamel prisms in the surface layer in atomic force (AFM) and inverted microscopes. — Patent RU № 2702903, effective from 14.03.2018 (In Russ.).